
Scalable Die-Cutting Solutions for Consumer Wearables
As an ISO 13485-certified medical contract manufacturer, JBC provides turnkey manufacturing for finished, retail-ready stick-to-skin products. We help consumer wearable OEMs and startups scale production by providing a U.S.-based supply chain that eliminates international shipping delays and quality inconsistencies.
As a vertically integrated medical contract manufacturer with Class 8 cleanroom capabilities, JBC helps consumer wearable OEMs and startups scale their design from prototype to production, utilizing our rapid prototyping capabilities and high-volume, value-added rotary die-cutting machines.
Transforming Medical-Grade Adhesives Into Lifestyle Wearable Products MOUTH TAPES, FASHION TAPES, WELLNESS STRIPS, WEARABLE DEVICE COVERS, AND MORE
|
From mouth tapes to nasal strips and acne patches, die-cut, medical-grade, and skin-safe adhesive tapes find their way into various consumer wearable applications. JBC is the key to turning these raw materials into their full functional potential. |
TABLE OF CONTENTS: |

Custom Manufactured Mouth Tapes
JBC Technologies provides high-volume contract manufacturing for over-the-counter mouth tapes and sleep-wellness brands. Using multi-layered lamination to integrate high-MVTR skin-safe adhesives from a range of industry material manufacturers, our ISO 13485 certified converting ensures safe removal and long-wear durability, from low-volume prototype to high-volume production.

Custom Manufactured Nasal Strips
JBC specializes in precision converting for skin-safe, breathable medical-grade adhesives for over-the-counter nasal strips and nose tapes. As a US-based ISO 13485 Certified nasal strip manufacturer with relationships with industry-leading materials suppliers, JBC has access to a wide range of skin-contact adhesives that ensure high MVTR-performance for sleep, sport, and wellness.

Custom Manufactured Breast Tapes
JBC Technologies is a contract manufacturer of breast tapes and nipple covers with extensive experience converting sweat-resistant tapes for athletics and running and medical-grade adhesives into functional, scalable breast and body tape solutions. From rapid prototyping to high-volume production, our process emphasizes long-term wear durability and skin compatibility for optimal performance and user experience.

Custom Manufactured Face Tapes
As an ISO 13485 Certified beauty tape manufacturer, our cosmetic die-cutting capabilities help brands convert thin, low-profile, skin-friendly hydrocolloids and adhesives into functional beauty/dermatological products optimized for easy removal and patient comfort. Through our VA/VE engineering support and Class 8 cleanroom capabilities, we ensure low particulate count for optimal skin-contact face lift tapes, under eye patches, acne patches, and more.

Custom US Manufactured Fashion Tapes
JBC Technologies optimizes the production of skin and clothing tapes through our vertically integrated die-cutting and converting capabilities, including the lamination of various double-sided adhesives to ensure an optimal balance between skin-adhesion and removal from textile surfaces. As a high-volume fashion tape manufacturer, JBC helps convert skin-safe medical tapes into functional, scalable fashion tape products.

Custom Manufactured Transdermal Patches
JBC provides precision die-cutting for nutraceutical and functional topical patches, featuring multi-layer island placement for active botanical elements. As an ISO 13485-certified manufacturer, we maintain rigorous quality standards for wellness and performance patches. Our vertically integrated process supports specialized labeling and custom packaging designed to preserve sensitive ingredients for retail distribution.

Custom Manufactured CGM Overlay Patches
JBC Technologies is an ISO 13485-certified manufacturer of custom OTC overlay patches for consumer wearables, including CGM patches for Dexcom G6/G7 and Abbott FreeStyle Libre.
We convert biocompatible, skin-safe adhesives into custom products optimized for 10-14 day wear. Our high-volume capabilities include adhesive "dead-zones," custom color printing, and part presentation features like scored liners and pull tabs. From bulk components to custom branded packaging, we provide turnkey, retail-ready solutions.
Strict Quality Management Systems For Over-the-Counter Skin Contact Adhesive Products
JBC Technologies is the only manufacturing partner you need for your functional body tape project, whether it be a wellness tape, a beauty patch, or something else entirely. With ISO 13485 Certification, a rigorous quality management system, various inline defect detection systems, and a vertically integrated supply chain, JBC is more than a medical contract manufacturer – we’re a scalable partner, from prototype to production.

ISO 13485 Certified For Medical-Grade Quality
JBC adheres to a rigorous ISO 13485 Quality Management System (QMS) that emphasizes traceability, comprehensive risk management, and stringent documentation standards to meet the stringent regulations of the medical and consumer wellness industry.

Controlled Environments ISO Class 8 Cleanrooms
Our ISO Class 8 cleanroom environments are a critical part of the safety and biocompatibility of consumer wearable components like skin-contact patches and adhesive sensors. By managing particulate levels and preventing contamination, our cleanrooms exemplify our commitment to meeting the highest quality standards for short, mid, and long-term wear consumer devices.

Rigorous Quality Control to Meet the Demanding Needs of the Consumer Market
To mitigate the risk of defective components, JBC implements a multi-level inline vision quality control system directly onto our high-volume rotary die-cutting machines. This real-time quality control system utilizes a good, better, best system to detect inconsistencies before they even leave the press.
Contract Manufacturing and Process Engineering Capabilities for Consumer Wellness Tapes
Here’s a breakdown of our consumer wearables converting toolkit…

Rapid Prototyping for Wear Studies & Design Validation
Rapid prototyping is a key capability for successfully launching consumer wellness products like mouth tapes, nasal strips, and transdermal patches, allowing you to quickly test materials, designs, skin compatibility, and more with no tooling investment. If we have the material on hand, we can even get prototypes in hand the same day.
Our digital cutting capabilities include:
- Multiple Atom flashcutters
- Eastman cutting tables
- Non-abrasive waterjet
- CO2 laser cutting
Our rapid prototyping services are designed to give you a responsive way to test different materials and designs quickly before jumping into high-volume production, helping you prototype quickly and scale even faster.

Multi-Layer Laminating for Increased Throughput
Through our in-line multi-layer laminating, we transform your stick-to-skin wellness tape product designs into scalable, high-yield realities. We optimize the "build" to ensure your specific performance needs are met with every part produced.
Process engineering-driven laminating capabilities include:
-
Thermal Bond Optimization: Utilizing heated nip rollers to achieve superior interlayer adhesion
-
Precision Slitting & Back-Scoring: Customizing the "kiss-cut" and liner presentation
-
Surface Preparation: In-line treatments to ensure low-surface-energy materials bond reliably
-
High-Yield Material Handling: From table-fed laminators for specialty runs to high-speed inline systems for maximum efficiency.
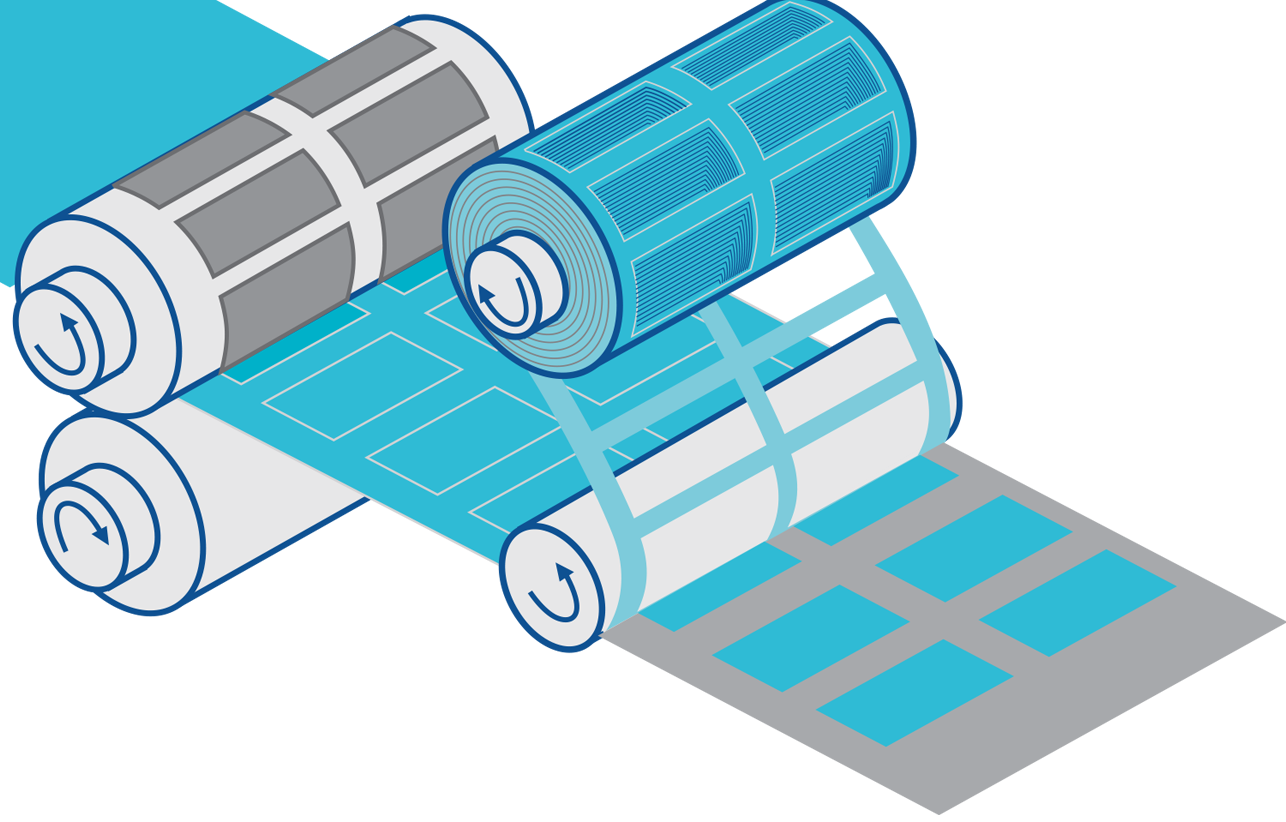
Rotary Die-Cutting For Complex Multi-Layer Products
JBC’s multi-station rotary presses transform complex wearable designs into high-volume reality. Our fleet ranges from high-speed mechanical analog to advanced 18-station servo-driven systems.
Our process engineering optimizes your production for reliability and scale:
-
Maximized Material Yield: Engineered die-cut paths reduce scrap and optimize nesting
-
Micron-Level Registration: CCD camera-guided systems align 10+ layers to eliminate assembly variability
-
Scalable Versatility: Our fleet handles everything from high-volume simple parts to intricate, multi-layered "stick-to-skin" wearables.
-
Enhanced User Experience: In-line back scoring creates "crack-and-peel" liners and pull-tabs, for effortless end-user application
Island Placement For Strategically Placed Layers
JBC’s in-line island placement creates precise, adhesive-free "dead zones" or active ingredient areas directly on stick-to-skin wearables. This ensures that medications or sensors stay isolated from the adhesive—preventing irritation, messy seepage, or evaporation.
Example applications include:
-
CGM Overlays: Adhesive-free centers for painless sensor removal.
-
Nasal & Lift Tapes: Strategic support that protects sensitive skin.
-
Wearable Devices: Multi-layered stability without compromising breathability.
Capabilities include gapping, tipping, vacuum transfer, low tack transfer, and stacked die transfer.

Packaging & Kitting For Retail Ready Products
From retail-ready branded packaging to industrial and automation-ready kitting, JBC streamlines your supply chain through a wide range of packaging and kitting capabilities. We provide vertically integrated assembly and packaging for face and wellness tape products that meet your post-processing requirements.
Our packaging capabilities include:
-
Consumer: Tins, boxes, pouches, sachets, bags, sleeves, and more
-
Industrial: Master cartons and overpacks
To determine which packaging solution is right for your specific consumer wellness tape product, discuss your post-processing needs and go-to-market plans with your converting partner!

Custom Color Printing for Designs and Instructions
JBC provides custom color printing and branding for consumer wellness tape designs and printed instructions. Using non-irritating, medical-grade, skin-safe inks, JBC prints your brand's design, helpful instructional information, and decorative patterns directly on the tape, fabric, or liner itself, ensuring your product is easy for the end user to apply and stands out on store shelves.
JBC’s in-house digital inkjet printing capabilities include:
- 10" wide printing radius
- CMYK print engine for full-spectrum color printing
- Resolutions of 600 and 1200 dpi
- Variable data printing
JBC is proud to work with leading medical adhesive manufacturers such as:
 |
 |
 |
Common Medical Grade Materials Used to Manufacture Wellness Tapes & Beauty Patches
Thanks to our years of experience with leading adhesive manufacturers and our own in-house adhesive experts, JBC Technologies can help you find suitable options for your consumer wearable project. JBC is proud to partner with some of the top medical adhesive manufacturers in the industry, maintaining status as both a Solventum Premier Converter and an Avery Dennison AdVantage Converter.
Here are some common materials we convert for consumer wearable projects:
Medical Grade Acrylic Tape
Typically used in long-term wear (3-28 day) applications, the high moisture vapor transmission rate (MVTR) of acrylic adhesive tapes makes them a great option for keeping devices secured in the face of sweat, water, or other bodily fluids/environmental factors.
- Key benefit: High shear and initial tack, High moisture vapor transmission rate (MVTR)
- Best used for: Multi-week wear applications including CGM overlay patches, wearable insulin pumps, long-term cardiac monitors, and "workout-proof" lifestyle patches
- Examples: Avery Dennison MED 5739 ; Solventum 4578
Silicone Adhesive Tapes
Silicone adhesive tapes offer more gentle adhesion and greater repositionability than acrylic adhesives, making them ideal for sensitive skin and atraumatic removal over medium wear duration (1-3 days).
- Key benefit: Constant adhesion levels, repositionability without loss of tack, and hypoallergenic properties that minimize redness and irritation.
- Best used for: Short-to-medium wear (1–3 days) where user comfort and skin integrity are the priority. Ideal for daily-use mouth tapes, gentle-release fashion strips, and "repositionable" wellness patches.
- Examples: 3M (Solventum) 2480
Hydrocolloid Adhesives
Hydrocolloids are special “absorptive” adhesives that create a moist healing environment while remaining waterproof for 1-5 days.
- Key Benefit: Remains waterproof while absorbing exudate
- Best used for: Medium-wear applications that require active fluid management. Ideal for acne/pimple patches, friction-reducing blister pads, and post-treatment skin recovery patches.
- Examples: Avery Dennison MED 5080H
Nonwoven Carriers
Nonwovens are a breathable, cloth-like material often utilized in conjunction with silicone and acrylic adhesives as a backer or carrier material. Thanks to their flexibility, nonwovens provide structural support while remaining bendable/stretchable.
- Key Benefit: Breathability, Flexibility, Structural integrity
- Best used for: Providing breathable structural integrity for acrylic & silicone adhesives used in mouth tapes, breast tapes, athletic body tapes
- Examples: https: Polyken 3621A Long-term Wear Medical Grade Non-woven Tape
Polyurethane (PU) Films
Polyurethane (PU) films are highly breathable, tear-resistant elastic films often used as a flexible “second skin” carrier material in consumer wearables.
- Key Benefit: Highly flexible, high moisture vapor transmission rate (MVTR), waterproof
- Best used for: High-movement applications that require a waterproof seal, such as CGM covers, workout-proof fashion tapes, and multi-day wellness patches.
- Examples: 3M (Solventum) Medical Film 9832F
Polyethylene (PE) Films
Polyethylene (PE) Films are more affordable but slightly less elastic and breathable than PU films, often used as a translucent carrier for disposable stick-to-skin wearable components.
- Key Benefit: Waterproof, translucent, chemically resistant, affordable
- Best used for: Best used for: Cost-effective, daily-wear products that need to be waterproof and discreet, such as disposable daytime mouth strips or clear fashion stays.
- Examples: Avery Dennison MED 3044
ENGINEERING Q&A:
Precision Die-Cutting and Material Selection for Consumer Wearables
Selecting the right long-term wear adhesive for consumer patches involves the consideration of a few different factors, including desired wear duration, device design, end-user demographics, environmental conditions, and performance requirements.
For nose strips and mouth tapes specifically, you’ll need an adhesive that is breathable, flexible, and sensitive enough for responsibility and safe removal while remaining adhered for the desired amount of time.
The key to selecting the right adhesive is consulting with a manufacturing partner like JBC Technologies early in the design process to ensure you’re specifying the proper material.
For a deeper dive on this adhesive selection, check out this helpful adhesive selection guide!
Although they’re often used interchangeably, “skin-safe” and “medical-grade” represent two distinct categories and regulatory levels of adhesives, each with its own distinct use cases.
“Skin-safe” adhesives are a broader classification, typically used on less demanding consumer-facing applications like fashion tape, skin contact beauty tapes, athletic tapes, sleep tapes, or mouth tapes. These adhesives are hypoallergenic and undergo basic testing to ensure they do not cause harm or irritation when placed on the skin. These are the go-to choices for shorter-term, less aggressive bonding.
“Medical-grade” adhesives are specifically designed and tested for use in higher-risk medical applications like continuous glucose monitor (CGM) patches, Holter monitors, and wound care. These adhesives are optimized for sensitive skin and must remain adhered for longer-durations of time, often withstanding sweat, water, and other environmental factors.
Think of “skin-safe” adhesives as the affordable adhesive for average skin-types, and “medical-grade” adhesives as specialized adhesives optimized for sensitive skin, long-term wear, and more “high-risk” applications.
While most “medical-grade” adhesives can be used in place of “skin-safe” adhesives, sometimes they may be overqualified for the job, unnecessarily driving up costs, and undermining the application. For help determining which adhesive is right for your consumer wearable project, be sure to consult your converter early in the design phase to avoid over-specifying materials.
JBC Technologies supports both low-volume prototyping and high-volume wholesale manufacturing for consumer wearables. JBC specializes in helping consumer wearables OEMs and startups scale their wearable designs from prototype to high-volume manufacturing.
Partnering with a U.S-based converting partner like JBC Technologies helps consumer wellness brands sidestep the unforeseen expenses of long-distance overseas supply chains, granting more consistent control over prices, quality, and lead times.
Notable benefits of a U.S.-based converting partner are:
-
Minimized inventory handling thanks to smaller, more frequent shipments (no overseas bulk orders!)
-
Faster lead times
-
Increased responsiveness to product changes and design defects
-
No fluctuating tariffs or fees due to the ever-changing geopolitical landscape
Partnering with a USA contact medical manufacturer is a hedge against global risk and an investment in supply chain security. Learn more about the impact of a US-based contract manufacturer here!
Yes, despite their name, medical-grade adhesives find their way into over-the-counter consumer wearables more than you might think. At the end of the day, it comes down to how your adhesive needs to perform…
Even though your consumer wellness device is labeled as a non-medical lifestyle or wellness product, a medical-grade adhesive might be doing the heavy lifting. For consumer applications that demand long-term wear durability, sweat and water resistance, and atraumatic removal on sensitive-skin, medical-grade adhesives are often the answer, providing the performance necessary that other non-medical “skin-safe” adhesives can’t provide. Think continuous glucose monitor (CGM) patches, heart monitors, and other wear-for-days devices. “Medical-grade” adhesives are often required to keep these devices secured through exercise, sweat, showers, and sleep.
The more affordable “skin-safe” non-medical adhesives typically associated with consumer wearables are great for short-term, less aggressive adhesion. For more information on the difference between “skin-safe” and “medical-grade” adhesives, see Q&A #2 above.
JBC leverages a wealth of die-cutting machinery and converting expertise to yield consistent, high-quality consumer and medical wearables.
Consumer wearable components are typically fabricated on one of our many high-volume rotary die-cutting machines with inline laminating, printing, and island placement capabilities. For components that require low-particulate counts, we have multiple ISO Class 8 Cleanroom environments to ensure particulate-free die-cutting.
Selecting the right material for your skin-contact consumer patch depends heavily on the performance goals of your product.
For mouth tapes, the goal is to create a gentle, yet secure bond for overnight or medium-length wear duration. Mouth tapes need to be breathable, conformable, and easily removed without skin irritation. Mouth tapes typically consist of a nonwoven carrier (like KT tape), which adds structural integrity and breathability, and a silicone adhesive for a secure adhesion that removes without damaging sensitive facial skin.
Although similar, nasal strips sometimes require more structural integrity and greater adhesion than mouth strips do, meaning nonwovens and silicone adhesives aren’t always applicable. For this, a slightly stronger acrylic adhesive and a more rigid Polyethene or PET liner may be necessary.
For more information on which material is right for your consumer wearable project, always consult with your manufacturer beforehand.
Yes, through our many vendor/supplier relationships, we have access to a wide range of both water-resistant materials with a high moisture vapor transmission rate that help absorb liquid.
Precision. Speed. Scale. Your Source for Scalable Consumer Wearable Solutions
As a wholesale consumer wearables supplier, JBC Technologies provides full-product lifecycle assistance from prototype to high-volume manufacturing. With an engineering-forward manufacturing approach, JBC employs design for manufacturability and assembly (DFMA) considerations wherever possible to help guarantee your wearable design can be successfully manufactured at scale.
With JBC’s facilities proudly based in the United States, this is critical for wearable tech startups and OEMs looking for a hedge against global instability and uncertainty, helping consolidate long overseas supply chains.
Interested in learning what an ISO 13485 certified medical contract manufacturer can do to make your wearable device component more manufacturable?